Synthesis of hexahydroquinoline (HHQ) derivatives using ZrOCl2·8H2O as a potential green catalyst and optimization of reaction conditions using design of experiment (DOE)†
Abstract
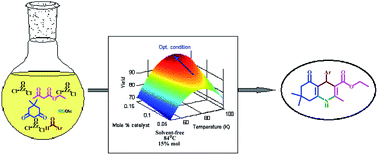
In this investigation, hexahydroquinoline (HHQ) derivatives were synthesized via a one-pot reaction using dimedone, β-ketoester, ammonium acetate, and different aryl aldehydes. ZrOCl2·8H2O was used as a potential green catalyst, it is a commercially available solid material, with low toxicity, low cost and high activity, and it is easy to handle. The reaction conditions were optimized using response surface methodology (Central Composite Design (CCD)) with three replicates at a central point. Optimization showed that the optimum reaction temperature and amount of catalyst are 83.75 °C and 0.15 mol%, respectively. The lower reaction yields at temperatures higher than 83.75 °C are related to the formation of a new crystalline phase of ZrOCl2·8H2O. The fitted quadratic polynomial model applied to the experimental yield could well predict the experimental reaction yield. Ecofriendly reaction conditions, easy workup procedure, the reusability of the catalyst, short reaction times and high yields are some of the advantages of this work.

 Please wait while we load your content...
Please wait while we load your content...