β-Cyclodextrin in water: highly facile biomimetic one pot deprotection of phenolic THP/MOM/Ac/Ts ethers and concomitant regioselective cyclization of chalcone epoxides and 2′-aminochalcones†
Abstract
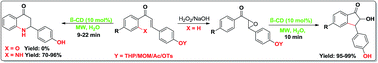
A mild and efficient one-pot deprotection of THP/MOM/Ac/Ts ethers and the concomitant cyclization of chalcone epoxides to 2-hydroxyindanones or 2′-aminochalcones to aza-flavanones using β-cyclodextrin in water has been developed. β-CD was found to be highly effective at carrying out the deprotection and sequential transformations in an eco-friendly environment affording moderate to excellent yields (59–99%) at 60 °C in 8–22 min. Water, an eco-friendly reaction medium, has been utilized for the first time in this reaction. The merits of the presented protocol include the high yields and catalyst reusability and the protocol precludes the use of metals and organic solvents. The present method is much milder but more advanced than those reported earlier.

 Please wait while we load your content...
Please wait while we load your content...