Yields of H2 and hydrated electrons in low-LET radiolysis of water determined by Monte Carlo track chemistry simulations using phenol/N2O aqueous solutions up to 350 °C
Abstract
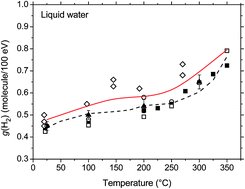
The effect of temperature on the yields of H2 and hydrated electrons (eaq−) in the low linear energy transfer (LET) radiolysis of liquid water has been modeled by Monte Carlo track chemistry simulations using phenol/N2O aqueous solutions from 25 up to 350 °C. N2O was used to scavenge eaq− and H˙ atoms formed in spurs giving N2 as a product. The primary aim of this work is to elucidate the main factors that account for the anomalous increase in the H2 yield with temperature. Comparing our calculated H2 and N2 yields with experiments led us to re-evaluate certain parameters involved in radiolysis, such as the H−/H2O dissociative electron attachment (DEA) cross section and its variation with temperature. Most importantly, we found that the prompt DEA process largely dominates the temperature dependence of the primary yield of H2 over most of the temperature range considered. Unlike what has been proposed by some authors in the literature, our simulations showed that the oxidation of water by H˙ atoms contributes only ∼12% of the total g(H2) at 350 °C and is thus insufficient to quantitatively explain, by itself, the increase in g(H2) with temperature that is observed experimentally above 200 °C.

 Please wait while we load your content...
Please wait while we load your content...