Solvent-controlled formation of four Ni(ii) coordination polymers based on a flexible bis(imidazole) ligand: syntheses, structural diversification, properties†
Abstract
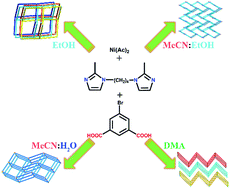
To further investigate the influence of solvent in the self-assembly process, four new solvent-controlled isomeric Ni(II) coordination polymers based bib and 5-Br-H2ip ligands, namely, [Ni(bib)(5-Br-H2ip)(CH3CH2OH)]n (1), [Ni(bib)(5-Br-H2ip)]n (2), {[Ni2(bib)(5-Br-H2ip)2(H2O)]·0.5H2O}n (3), {[Ni(bib)(5-Br-H2ip)]·DMA}n (4) (bib = 1,4-bis(2-methylimidazol-1-yl)butane, 5-Br-H2ip = 5-bromoisophthalic acid) were prepared under the same reaction conditions except with diverse solvent systems. Their EA, IR, PXRD, single crystal XRD analysis and TG analysis have been characterized. As controlled different solvent systems, complex 1 exhibits an unusual [2 + 2] dia construction with the point symbol 66. Complex 2 shows a 3D non-interpenetrated dia structure with the point symbol 66. Complex 3 features a dinuclear 3D pillar-layer pcu network with the point symbol of 412·63. Complex 4 displays a 2D stacked layer sql net with the point symbol 44·62. The results demonstrate that solvent has a significant effect on the final structure of coordination polymers. Furthermore, the magnetic properties of complexes 1–4 have also been studied.

 Please wait while we load your content...
Please wait while we load your content...