Termini capping of metal-poly-His peptide complexes induces the formation of α-helix†
Abstract
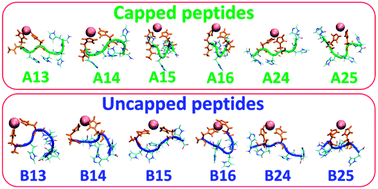
An ensemble of structures of metal-hexa-histidine-tag capped and uncapped peptides has been studied using molecular dynamics simulations. The metal-binding peptides are polymorphic for both capped and uncapped peptides. The capping of the peptide termini promotes α-helical conformations that are induced by the metal binding sites.

 Please wait while we load your content...
Please wait while we load your content...