(−) and (+)-Merrilliaquinone, a pair of new quinone enantiomers from Illicium merrillianum and their distinctive effect on human hepatoma and hepatic cells
Abstract
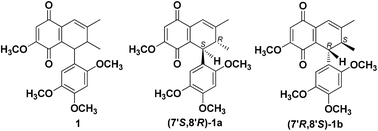
Merrilliaquinone (1), a new racemic quinone was isolated from the branches and leaves of Illicium merrillianum. Chiral separation of 1 gave two enantiomers (−)-merrilliaquinone (1a) and (+)-merrilliaquinone (1b). The structure of 1 was established by comprehensive spectroscopic analysis, and the absolute configurations of 1a and 1b were determined by quantum mechanical calculation of ECD spectra. It is very interesting that 1b had a selective cytotoxicity against human hepatoma cell lines SMMC7721 and HuH7 with IC50 values of 0.91 μM and 1.29 μM, while its IC50 values on normal human hepatic cells QSG7701 and LO2 were 47.79 μM and 36.71 μM, respectively. Moreover, 1a and racemate 1 only exhibited very weak cytotoxicity against SMMC7721 and HuH7 cells with IC50 values of 27.01–37.82 μM. These results implied that the absolute configurations of 1a and 1b possess remarkable influences on their cytotoxicities. Further mechanism studies indicated that 1b dose-dependently induced SMMC7721 cell apoptosis and reduction of mitochondrial membrane potential (MMP) at 1–10 μM. Flow cytometry analysis showed that the 1b-induced SMMC7721 cell apoptosis was associated with cell cycle arrest during the G0/G1 phase.

 Please wait while we load your content...
Please wait while we load your content...