Insight into the structural stability of coumestrol with human estrogen receptor α and β subtypes: a combined approach involving docking and molecular dynamics simulation studies†
Abstract
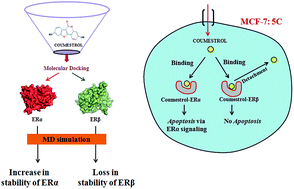
Epidemiological studies suggest that dietary consumption of phytoestrogens is associated with a lower risk of breast cancer. Among phytoestrogens, coumestrol employs estrogen receptor (ER) as a target to induce apoptosis in cancer cells. Competitive binding experiments revealed a higher affinity of coumestrol for ERβ than for ERα. However, recent evidence demonstrates that apoptotic potential of coumestrol in breast cancer cells requires ERα and not ERβ. It was, therefore, pertinent to enhance our understanding of coumestrol selecting ERα or ERβ subtype. In the present study, we elucidated the binding mechanism of coumestrol to ERα and ERβ at a molecular level using molecular docking, access channel analysis and molecular dynamics (MD) simulations. The MD approach was used to determine the structural stability of coumestrol docked to ERα and ERβ by analysing H-bonds, interaction energy, radius of gyration, solvent-accessible surface area, root mean square deviation (RMSD), RMS fluctuation and secondary structure elements. Our results clearly suggest that coumestrol on interaction with ERβ causes an overall destabilization of Apo–ERβ structure whereas the same on interaction with ERα leads to strong substrate binding and an increase in Apo–ERα structural stability. Principal component analysis revealed higher strenuous motions of the coumestrol–ERβ complex further supporting destabilization of coumestrol–ERβ during the MD run. In conclusion, this is the first report in which in silico approaches were implemented to suggest the effect of structural stability on selective binding of coumestrol to ERα and not to ERβ. We expect these findings to provide significant insights into ER-based drug development particularly for receptor mediated mechanisms for breast cancer treatment.


 Please wait while we load your content...
Please wait while we load your content...