Synthesis of tetra-substituted olefins via annulation by Pd-catalyzed carbopalladation/C–H activation and solid state fluorescence properties†
Abstract
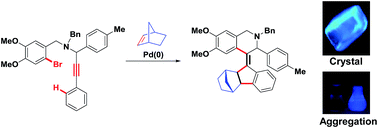
Palladium-catalyzed and norbornene-mediated multiple C–C bond forming strategies to highly substituted helical olefin incorporated 1,2,3,4-tetrahydroisoquinolines have been developed via double carbopalladation/C–H activation of 2-bromo-N-benzylpropargylamines. The scope of the above carbocyclization process has been studied by varying the substitution on starting materials which have been synthesized using A3-coupling (![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) mine–
mine–![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) ldehyde–
ldehyde–![[a with combining low line]](https://www.rsc.org/images/entities/char_0061_0332.gif) lkyne). Interestingly, the synthesized compounds show a pronounced solid state fluorescence property due to their restriction of intramolecular rotation in the condensed phase.
lkyne). Interestingly, the synthesized compounds show a pronounced solid state fluorescence property due to their restriction of intramolecular rotation in the condensed phase.

 Please wait while we load your content...
Please wait while we load your content...