Novel aromatic polyimides derived from 2,8-di(3-aminophenyl)dibenzofuran. Synthesis, characterization and evaluation of properties†
Abstract
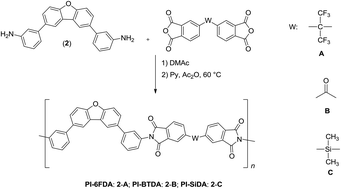
Three aromatic polyimides (PIs) were prepared from a new aromatic diamine monomer derived from the rigid ring dibenzofuran. All PIs were obtained in high yield and the inherent viscosities were in the range of 0.60 and 0.74 dL g−1. Polyimides derived from 4,4′-hexafluoroisopropyliden diphthalic anhydride (6FDA) and 4,4′-(dimethylsilanediyl) diphthalic anhydride (SiDA) showed excellent solubility in a variety of aprotic polar organic solvents. All PIs showed high thermal stability with thermal decomposition temperature (TDT10%) between 555–590 °C and the glass transition temperatures (Tg) values were between 290 and 315 °C. Polymeric films were obtained from PI-6FDA and PI-SiDA solutions and then contact angle and surface free energy were tested in order to know the hydrophobicity of materials. Likewise, permeability and selectivity analyses were developed where PI-6FDA film offered a reasonably acceptable balance of permselectivity with values close to the Robeson upper-bound (1991), in particular for the CO2/CH4 gas pair.

 Please wait while we load your content...
Please wait while we load your content...