Iodine-mediated regioselective C–N and C–I bond formation of alkenes†
Abstract
Iodine-mediated intermolecular C–N and C–I bond formation of alkenes is described. A series of alkenes could be selectively converted into the corresponding aminoiodination products, which are versatile building blocks in organic synthesis and medicinal chemistry. Wide substrate scope, broad functional group tolerance and easy scale-up operation make this protocol very practical and attractive in synthetic chemistry.
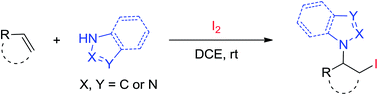

 Please wait while we load your content...
Please wait while we load your content...