Green synthesis of (R)-3-TBDMSO glutaric acid methyl monoester using Novozym 435 in non-aqueous media†
Abstract
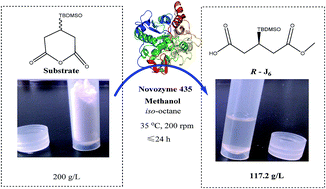
An efficient biocatalytic synthesis of (R)-3-TBDMSO glutaric acid methyl monoester (R-J6), an important intermediate in the synthesis of rosuvastatin, has been developed using a green catalytic route in the presence of lipase, conducted under mild conditions without additional chiral reagents. Enzyme screening indicated Novozym 435 to be the most efficient biocatalyst for R-J6 synthesis. Methanol, which was the most effective alcohol for synthesis of R-monoester, was identified as the best acyl acceptor by molecular docking. The optimal conditions for synthesis of R-J6 were as follows: 50 g L−1 catalyst, 3 : 1 molar ratio of methanol : substrate, 200 g L−1 substrate, iso-octane as solvent, orbital shaking at 200 rpm, and an incubation time of 24 h at 35 °C. The key factor affecting the yield of R-J6 was the molar ratio of methanol to substrate found by an orthogonal array experimental design. Consequently, the desired product, R-J6, was afforded with a titer of 117.2 g L−1, a yield of 58.6%, and productivity of 4.88 g L−1 h−1. This green method holds promise for the preparation of kilogram quantities of (R)-3-substituted glutaric acid monoesters.

 Please wait while we load your content...
Please wait while we load your content...