Insights into the epimerization activities of RaCE and pAGE: the quantum mechanics/molecular mechanics simulations†
Abstract
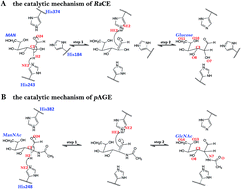
Ruminococcus albus cellobiose 2-epimerase (RaCE) and N-acetyl-D-glucosamine 2-epimerase from porcine kidney (pAGE) belong to the AGE superfamily and have a detectable AGE activity. Interestingly, RaCE and pAGE share a set of conserved residues and have a similar catalytic mechanism, although they exhibit different substrate specificities, in that RaCE reacts with unmodified oligosaccharides and pAGE reacts with modified monosaccharides. In this study, the reaction mechanisms of RaCE and pAGE were theoretically calculated by using the quantum mechanics/molecular mechanics method based on the umbrella sampling simulations. The simulation results not only characterize the detailed mechanism of enzyme actions, but also reveal the difference between the catalytic mechanism of RaCE and pAGE. The energy barriers of the two reactions indicate that the activity of reprotonation in the epimerization activities is much higher than that of the deprotonation. Additionally, we obtained the structural information of the transition states of deprotonation and reprotonation and the stable intermediate states and analyzed the enzyme function of lowering the activation energy. Our work can provide important information to understand the catalytic mechanism of the AGE family.

 Please wait while we load your content...
Please wait while we load your content...