Electrochemical performance degeneration mechanism of LiCoO2 with high state of charge during long-term charge/discharge cycling†
Abstract
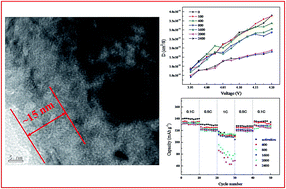
Electrochemical performance degeneration of LiCoO2 electrodes under high state of charge (SOC) during long-term cycling was studied using LiCoO2/MCMB batteries. The batteries were charged/discharged at 0.6C with 30% depth of discharge (DOD) for 100, 400, 800, 1600, 2000 and 2400 cycles, respectively, and then disassembled to analyze the evolution of morphology, element content, microstructure and electrochemical performance. Through energy dispersive spectrometer (EDS), Fourier transform infrared spectroscopy (FT-IR), X-ray photoelectron spectroscopy (XPS) and high resolution transmission electron microscopy (HRTEM) characterization, it was confirmed that the formation of discontinuous solid electrolyte interface (SEI) layer consisting of Li2CO3, RCOOLi and LiF led to the increase of electrochemical charge transfer resistance (Rct). Although the X-ray diffraction (XRD) refined results showed that there was no new phases were formed during the long-term cycling, the actually increased Li/Co exchange ratio of LiCoO2 from 1.6% at 800th to 2.1% at 2400th resulted in the decrease of lithium ion diffusion coefficient and deterioration of the rate performance.

 Please wait while we load your content...
Please wait while we load your content...