Efficient absorption of ammonia with hydroxyl-functionalized ionic liquids†
Abstract
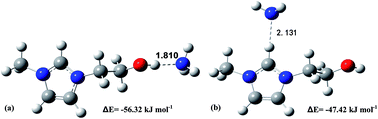
Ammonia (NH3) emitted from the ammonia synthesis process is a kind of waste chemical resource and a major environmental pollutant. The traditional water scrubbing method suffers from high energy consumption due to the concentrated NH3 from aqueous ammonia. Therefore, it is desirable to develop novel absorbents for the efficient, reversible and environmentally-friendly recovery of NH3. In this paper, a series of hydroxyl-functionalized imidazolium ILs ([EtOHmim]X, X = [NTf2], [PF6], [BF4], [DCA], [SCN] and [NO3]) were designed and prepared. Their physical properties and NH3 absorption capacities under different temperatures and pressures were systematically investigated. The effects of hydroxyl cation, anionic structures, pressure and temperature on absorption performance were sufficiently studied. In addition, the absorption mechanism was investigated in detail by spectral analysis and quantum chemistry calculations. Compared with conventional IL [Emim]X, a higher absorption capacity was achieved by introducing the hydroxyl group on the imidazolium cation. The mechanism results showed the fascinating absorption performance of the task-specific ILs was attributed to the stronger hydrogen bonding interaction between NH3 and the H atom of the hydroxyl group. Considering the excellent absorption performance, high thermal stability, and super reversibility, this type of IL provides great improvement over conventional IL and shows their enormous potential in NH3 recovery.

 Please wait while we load your content...
Please wait while we load your content...