Pd-catalyzed isocyanide insertion/nucleophilic attack by indole C-3/desulfonylation in the same pot: a direct access to indoloquinolines of pharmacological interest†
Abstract
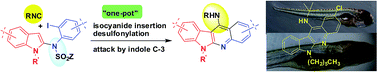
A new, simple and straightforward cascade reaction involving the Pd-catalyzed isocyanide insertion followed by a nucleophilic attack by the indole C-3 has been developed. The methodology afforded a diverse and unique class of indolo[2,3-b]quinolin-11-amines as potential cytotoxic agents some of which were evaluated in zebrafish.

 Please wait while we load your content...
Please wait while we load your content...