Study of the structure-activity relationship of flavonoids based on their interaction with human serum albumin†
Abstract
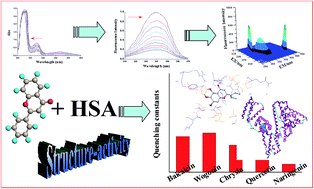
Structure-activity relationship (SAR) study helps in understanding biological effects of a compound, thus contributing to the development of new drugs. On the other hand, the study of protein-drug interaction is very important for understanding the mechanism behind versatile bioactivities of drugs. Flavonoids have been known for their numerous biological activities. To examine the potential of flavonoids as therapeutics, we systematically investigated the SAR of flavonoids based on their interaction with human serum albumin (HSA). Our study demonstrates that all the studied flavonoids (baicalein, wogonin, chrysin, naringenin, and quercetin) bind to HSA at the subdomain IIIA. Molecular docking was employed to investigate binding sites and the surrounding environment of flavonoids on HSA. We found that the number and position of hydroxyl groups, conjugated structure, and functional groups are responsible for differences in the interactions between different flavonoids and HSA. Our results together provide further molecular level understanding of protein–polyphenol binding and a strategy for SAR studies.

 Please wait while we load your content...
Please wait while we load your content...