Uptake of Nd3+ and Sr2+ by Li–Al layered double hydroxide intercalated with triethylenetetramine-hexaacetic acid: kinetic and equilibrium studies
Abstract
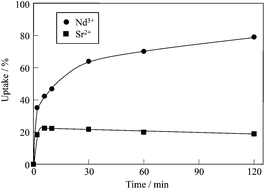
A Li–Al layered double hydroxide intercalated with triethylenetetramine-hexaacetic acid (TTHA·Li–Al LDH) was prepared by the drop-wise addition of an Al solution to a Li–TTHA solution at a constant pH of 8.0. TTHA·Li–Al LDH was found to take up Nd3+ and Sr2+ ions from aqueous solutions. This can be attributed to the metal chelating functions of the TTHA species in the interlayer of TTHA·Li–Al LDH, i.e., Nd–TTHA and Sr–TTHA complexes were formed in the interlayers. TTHA·Li–Al LDH was found to take up Nd3+ ions preferentially over Sr2+ ions from aqueous solutions, which can be attributed to the higher stability of the Nd–TTHA complex than Sr–TTHA. The mass-transfer-controlled shrinking core model described the uptake behavior better than the surface reaction-controlled model. The TTHA ions in the TTHA·Li–Al LDH interlayer quickly formed chelate complexes with Nd3+ or Sr2+, as a result of which the transfer rate of Nd3+ or Sr2+ through the product layer was rate limiting. Furthermore, this reaction was suitably represented by the Langmuir-type adsorption mechanism, indicating that it involves chemical adsorption, which is consistent with the formation of chelate complexes between Nd3+ or Sr2+ and TTHA ions in the interlayers of TTHA·Li–Al LDH.

 Please wait while we load your content...
Please wait while we load your content...