Electrochemical behavior of La(iii) on liquid Bi electrode in LiCl–KCl melts. Determination of thermodynamic properties of La–Bi and Li–Bi intermetallic compounds
Abstract
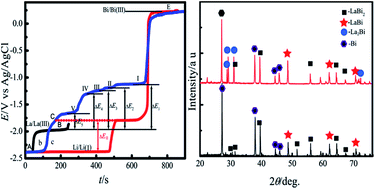
Electrochemical behavior of La(III) was studied on liquid Bi electrodes (i.e. Bi pool and Bi coated W electrodes) in LiCl–KCl melts using cyclic voltammetry, square wave voltammetry, chronopotentiometry and open circuit chronopotentiometry. In both the electrodes, the electrochemical reduction of La(III) was observed at more positive potential values than that on an inert W electrode, due to the lowering of the activity of La in liquid Bi phase. Cyclic voltammogram, using a Bi pool electrode, suggests that the reduction of La(III) to Lain liquid Bi was a quasi-reversible and diffusion controlled process. The diffusion coefficient of La in liquid Bi metal was measured by chronopotentiometry at 773 K and was evaluated by using the Sutherland–Einstein equation, respectively. Both the values of the diffusion coefficient were of the same order of magnitude. From the cyclic voltammogram and square wave voltammogram obtained on the Bi coated W electrode, five couples of cathodic/anodic peaks were observed at more positive potential than that for La metal on an inert electrode due to the formation of five La–Bi intermetallic compounds. Thermodynamic properties such as activities and relative partial molar Gibbs energies of M (M = La, Li) in the M–Bi alloys as well as Gibbs energies of formation for M–Bi (M = La, Li) intermetallic compounds, LaBi2, LaBi, La4Bi3, La5Bi3, La2Bi and Li3Bi were calculated from the open circuit potential measurement. La–Bi and La–Li–Bi alloys were produced on liquid Bi pool electrodes by galvanostatic and potentiostatic electrolysis, and characterized by X-ray diffraction (XRD) and scanning electronic microscopy (SEM). The results indicated that La–Bi alloys were comprised of LaBi2, LaBi and La2Bi phases, and LaBi2, LaBi and Li3Bi phases existed in La–Li–Bi alloys.

 Please wait while we load your content...
Please wait while we load your content...