N-Urethane protection of amines and amino acids in an ionic liquid†
Abstract
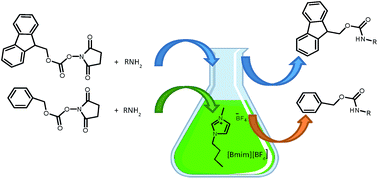
An efficient, solvent-free protocol for the N-fluorenylmethoxycarbonylation and N-benzyloxycarbonylation of amines is described. The reaction of aliphatic and aromatic amines with FmocOSu and Cbz-Osu in [Bmim][BF4] at room temperature afforded the corresponding N-urethane derivatives in excellent yields and do not require any further purification. The method has been extended to the N-Fmoc and N-Cbz protection of amino acids. Absence of bases, very short reaction times, high yields, selectivity and ease of product separation are some advantages of this protocol.

 Please wait while we load your content...
Please wait while we load your content...