Insights into the mechanism and kinetics of the gas-phase atmospheric reaction of 9-chloroanthracene with NO3 radical in the presence of NOx†
Abstract
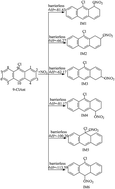
9-Chloroanthracene (9-ClAnt), an important member of the chlorinated polycyclic aromatic hydrocarbons (ClPAHs), has been demonstrated to show strong direct mutagenic effects. To elucidate the chemical transformations, degradation products and the capacity to undergo long-range transport of 9-ClAnt in the atmosphere, we conducted a theoretical investigation into the oxidation mechanism and kinetics of the NO3-initiated atmospheric transformation of 9-ClAnt by using a quantum chemistry method. The rate constants for the crucial elementary reactions were also estimated. The main oxidation products for the gas-phase reactions of 9-ClAnt with NO3 radicals include 9-chloroanthracen-yl nitrates, 9-chloroanthracenesdiones, epoxides, dialdehydes, 9-chloroanthracene-1,4-dione, anthracene-9,10-dione, 9-chloroanthracen-1-one, 10-chloroanthracen-1-one, 10-chloroanthracen-9-ol and 10-chloro-1-nitroanthracene etc. The overall rate constant of the NO3 addition reactions is 9.11 × 10−13 cm3 molecule−1 s−1 at 298 K and 1 atm. The atmospheric lifetime of 9-ClAnt determined by using NO3 radicals is about 0.61 h. This comprehensive investigation is the first report for the NO3-initiated oxidation of ClPAHs in the atmosphere. It should be conducive to clarifying their atmospheric fates and developing a full understanding of their toxic potential.

 Please wait while we load your content...
Please wait while we load your content...