Design and synthesis of conformationally homogeneous pseudo cyclic peptides through amino acid insertion: investigations on their self assembly†
Abstract
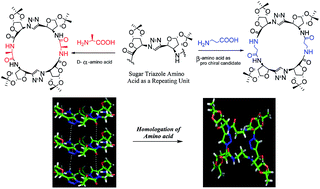
Macrocyclic C2 symmetric peptides, containing bis furanoid triazole amino acids as di-β-peptides linked to a D-α-amino acid or a β-amino acid in each half, have been designed and synthesized. The D-α-amino acid derived product undergoes parallel homo-stacking in solution via amide NH and amide carbonyl oxygen H-bonding; such macrocycles may be used as model systems for artificial ion channels as their unidirectional assembly pattern attributes them with large dipole moments. In contrast, the β-amino acid based compound forms only a conformationally homogeneous cyclic peptide without undergoing self assembly.

 Please wait while we load your content...
Please wait while we load your content...