Chemical regeneration mechanism of Fe-impregnated chitosan using ferric chloride†
Abstract
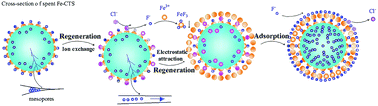
A process for the regeneration of spent Fe-impregnated chitosan (Fe-CTS) by continuous fluoride adsorption and chemical regeneration is described. Different concentrations of NaCl, FeCl3 and CaCl2 solutions were employed as regenerants. The regeneration efficiencies were valued using 100 mg L−1 fluoride adsorption on regenerated Fe-CTS. Results showed that the maximum adsorption capacity (qe) of 14.44 mg g−1 was obtained when the Fe3+ regenerant concentration was 150 mg L−1. Changes in F− and Cl− concentration were also measured. To examine the stability of regenerated Fe-CTS, Fe3+ leaching was observed in the fluoride solution after adsorption. Scanning electron microscopy with an electronic differential system and surface charge distribution were performed to elucidate the regeneration and adsorption mechanisms. Holes and cracks that emerged after regeneration accelerated the rate of internal diffusion. After regeneration, the FeCl3 solution pH was less than pHpzc (4.92), indicating that FeCl3 regenerated Fe-CTS (FeCl3-Fe-CTS) was positively charged. The change in the concentration of fluoride was consistently greater than that of chloride, indicating that other mechanisms except ion-exchange, such as electrostatic attraction, contributed to fluoride adsorption. After seven regeneration/adsorption cycles, the total adsorption capacity of FeCl3-Fe-CTS was found to be 74.04 mg g−1 without a significant loss in the adsorption ability.

 Please wait while we load your content...
Please wait while we load your content...