Influence of alkenyl structures on the epoxidation of unsaturated fatty acid methyl esters and vegetable oils†
Abstract
Epoxidation of vegetable oils or fatty acid methyl esters (FAMEs) produce important monomers which are widely used as plasticizers or stabilizers in the polymer industry. However, little attention has been focused on the influence of the alkenyl structure of the fatty acid on the efficiency and selectivity of their epoxidation. In this work, the influence of the alkenyl structure (the number of double bonds) of the FAMEs on the epoxidation reaction has been investigated. Three model FAMEs with 1 to 3 double bonds were epoxidized using both a weak (formic acid) and a strong (sulfuric acid/acetic acid) acid system. It was found that FAMEs with more double bonds have higher reactivities toward the epoxidation reaction. In addition, the electron-donating effect of the double bonds on the fatty acid chain tends to stabilize the resulting epoxide adjacent to it with the weak acid system. Furthermore, FAMEs with more double bonds easily undergo side reactions with the strong acid system (H2SO4). Epoxidation of two vegetable oils with different fatty acid compositions were carried out with the same two acid catalyst systems. And the results were in agreement with those from the FAMEs. The current findings could provide useful guidance for the epoxidation of different vegetable oils with different alkenyl structure compositions.
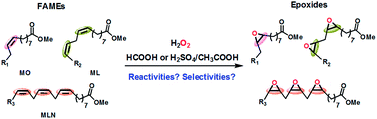

 Please wait while we load your content...
Please wait while we load your content...