A catalyst-free rapid, practical and general synthesis of 2-substituted quinazolin-4(3H)-ones leading to luotonin B and E, bouchardatine and 8-norrutaecarpine†
Abstract
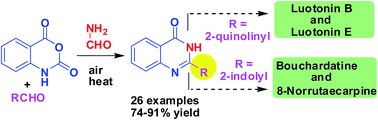
A remarkably rapid but microwave/ultrasound/catalyst-free method has been developed for the construction of a quinazolin-4(3H)-one ring using formamide as an efficient ammonia precursor and PEG-400 as an effective solvent. The methodology afforded various 2-substituted quinazolin-4(3H)-one derivatives in good yield via a three-component reaction of isatoic anhydride, aldehydes and formamide in air. This single methodology was extended successfully to the synthesis of several alkaloids e.g. leutonin B and E, bouchardatine and 8-norrutaecarpine.

 Please wait while we load your content...
Please wait while we load your content...