Probing the binding mechanism of novel dual NF-κB/AP-1 inhibitors by 3D-QSAR, docking and molecular dynamics simulations†
Abstract
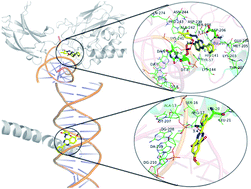
Nuclear factor-κB (NF-κB) and activator protein-1 (AP-1) are promising targets for a number of immunoinflammatory diseases, including asthma, psoriasis, rheumatoid arthritis, and transplant rejection. In this study, based on a dataset consisting of 127 pyrimidine/quinazoline-based derivatives as dual NF-κB/AP-1 inhibitors, an integrated computational protocol, including the three-dimensional quantitative structure–activity relationship (3D-QSAR), molecular docking and molecular dynamics (MD) simulations, was performed to explore the influence of the structural features on the NF-κB and AP-1 inhibitory activities and design derivatives with improved potency. The obtained CoMFA (comparative molecular field analysis) model exhibited satisfactory internal and external predictability. The most probable binding sites of the two receptors have been identified by docking and MD simulations, showing that they just locate on the joint regions between NF-κB (or AP-1) and DNA, wherein inhibitors can effectively prevent free NF-κB (or AP-1) from binding to DNA. At the same time, the key residues/deoxynucleotides for achieving strong binding were also revealed by docking studies, and the detailed dynamic binding process and binding modes of the inhibitors with different activities were determined by MD simulations. The binding free energies are in good agreement with the experimental bioactivities. The decomposition of binding free energies by MM-GBSA suggests that the hydrophobic interactions play an important role for the binding of compounds to NF-κB and AP-1. The results presented here can provide significant insight into the development of novel potential dual NF-κB/AP-1 inhibitors.

 Please wait while we load your content...
Please wait while we load your content...