Retracted Article: Simultaneous sorption and reduction of U(vi) on magnetite–reduced graphene oxide composites investigated by macroscopic, spectroscopic and modeling techniques
Abstract
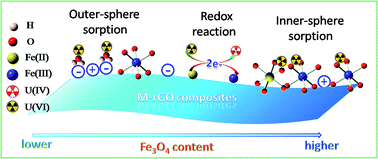
Magnetite–reduced graphene oxide (M–rGO) composites with different mass% (from 33% to 93%) magnetite contents were successfully synthesized via an in situ chemical precipitation method. The composites of M–rGO were characterized by SEM, XRD, FTIR, and XPS techniques. Macroscopic, spectroscopic and modeling techniques were used to study the mechanism for U(VI) removal by M–rGO. The results revealed that the high performance of M–rGO toward U(VI) removal resulted from the contribution of both sorption and reduction mechanisms. The reduction of U(VI) to U(IV) by M–rGO increased with increasing content of magnetite (Fe3O4), as evidenced by the XPS analysis. The kinetics model further suggested that the reduction reaction happened after the sorption of U(VI) on M–rGO. U(VI) was adsorbed on M–rGO via outer-sphere and inner-sphere surface complexation with oxygen-containing groups, whereas the inner-sphere surface complexation dominated with the increasing content of Fe3O4 on M–rGO due to the increased sorption sites (i.e., ![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) FeOH). The findings herein highlight the elucidation of the interaction mechanisms between M–rGO and U(VI), which is of significance in predicting the U(VI) removal properties of M–rGO and designing versatile adsorbents in environmental cleanup.
FeOH). The findings herein highlight the elucidation of the interaction mechanisms between M–rGO and U(VI), which is of significance in predicting the U(VI) removal properties of M–rGO and designing versatile adsorbents in environmental cleanup.

 Please wait while we load your content...
Please wait while we load your content...