Low temperature synthesis and mechanism of finely dispersed nanorod rutile titanium dioxide†
Abstract
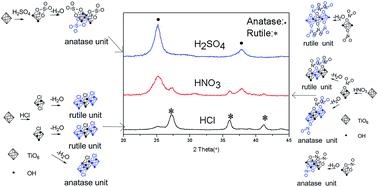
As a kind of one-dimensional (1D) nanostructured material, nanorod rutile titanium dioxide (TiO2) with high surface-to-volume ratio shows potential applications in UV-ray shielding, self-cleaning devices, energy conversion and storage. Well dispersed rod-shaped rutile was prepared by hydrolysis of TiCl4 with hydrochloric acid (HCl) solutions and FeCl3 at low temperature. The crystalline form and morphology were characterized by X-ray diffraction (XRD), Raman spectrum and transmission electron microscopy (TEM). The doping of iron was confirmed by X-ray photoelectron spectroscopy (XPS) analysis. A series of experiments were conducted to figure out the effects of reaction conditions on the crystalline form and shape of the powders. The results showed that either change of acids (H2SO4 or HNO3) or varying of cations (NaCl or AlCl3) leaded to a mixed products or pure anatase. A possible mechanism was proposed to explain why the change of minerals or acids would make such a remarkable difference.

 Please wait while we load your content...
Please wait while we load your content...