Ammonia borane in an external electric field: structure, charge transfer, and chemical bonding†
Abstract
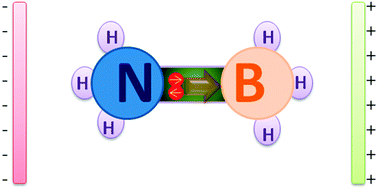
It is well known that ammonia borane (BH3NH3) is one of the simplest donor–acceptor complexes. The donor–acceptor bond (B–N bond) is formed by sharing lone pair electrons between BH3 and NH3 groups. In the present work, different strengths of external electric fields (Eext) are applied along the Z-axis direction to investigate the electric field induced effect on the BH3NH3 structure and properties. Interestingly, we have found that the lone pair electrons can be gradually moved in the “channel” between the BH3 and NH3 groups by modulating Eext. The donor–acceptor bond (B–N bond) is gradually elongated until it breaks when the Eext ranges from 0.0000 to 0.0321 au. The B–N bond is the shortest at Eext = −0.0519 au. Interestingly, the negative charge on BH3 groups sharply decreases from 0.161 to 0.005 (for NH3 groups decreases from 0.161 to 0.005) and the electron cloud of HOMO−2 exhibits an obvious transformation at “broking bonding” Eext ranging from 0.0320 to 0.0321 au, indicating the electron movement induced by the electric-field is the main reason to change the structure and stability of BH3NH3. Further, atoms in molecules (AIM) analysis shows that the B⋯N interactions are similar to that of hydrogen bonds at Eext = −0.0767 au and the iconicity of B–N bond in BH3NH3 is confirmed by its low electron density for B–N ρ(B–N) (0.103–0.073) in the region of Eext (0–0.025 au).

 Please wait while we load your content...
Please wait while we load your content...