Fabrication of high power LiNi0.5Mn1.5O4 battery cathodes by nanostructuring of electrode materials†
Abstract
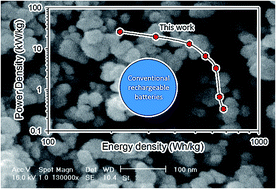
Using nanoparticles, instead of microparticles, as active electrode materials in lithium ion batteries could provide a solution to slow charging rates due to long ion diffusion pathways in conventional bulk materials. In this work, we present a new strategy for the synthesis of high purity lithium nickel manganese oxide (LiNi0.5Mn1.5O4) nanoparticles as a high-voltage cathode. A sonochemical reaction is used to synthesize nickel hydroxide and manganese dioxide nanoparticles followed by a solid-state reaction with lithium hydroxide. The product shows a single spinel phase and uniform spherical nano-particles under the appropriate calcination conditions. The LiNi0.5Mn1.5O4 exhibits a high voltage plateau at about 4.7–4.9 V in the charge/discharge process and delivers a discharge capacity of more than 140 mA h g−1 and excellent cycling performance with 99% capacity retention after 70 cycles. The synthesized nano-particles show improved electrochemical performance at high rates. This electrode delivers a power density as high as 26.1 kW kg−1 at a discharge rate of 40 C. This power performance is about one order of magnitude higher than traditional lithium ion batteries. These findings may lead to a new generation of high power lithium ion batteries that can be recharged in minutes instead of hours.

 Please wait while we load your content...
Please wait while we load your content...