Effect of different solvent ratio (ethylene glycol/water) on the preparation of Pt/C catalyst and its activity toward oxygen reduction reaction†
Abstract
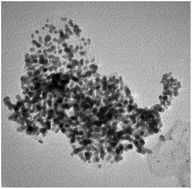
Highly dispersed 50 wt% Pt nanoparticles supported on carbon were successfully synthesized via a simple modified polyol method using different solvent volume ratios (ethylene glycol/water). The control of water proportion is found to have an important effect on the physicochemical properties of the in-house synthesized Pt/C catalysts. The structural and electrochemical characterizations reveal that the addition of water not only favors the homogeneous distribution of Pt particles with reduced particle size but also improves the electrocatalytic properties. In particular, at an optimum volume ratio of ethylene glycol to water of 45 : 23 among all studied ratios, the TEM images indicate that the as-synthesized Pt/C catalyst has a minimum particle size of 2.2 ± 0.5 nm, which is close to that of the commercial 46.6 wt% Pt/TKK catalyst, while the electrochemical measurements disclose electrochemical active surface areas and oxygen reduction reaction activity comparable to those of the commercial 46.6 wt% Pt/TKK catalyst. The simplicity of the synthesis process associated with the ease of the filtering step due to the addition of water has significant implications for its practical application in large scale production of Pt/C catalysts for polymer electrolyte membrane fuel cells.

 Please wait while we load your content...
Please wait while we load your content...