Highly efficient synthesis of amides from ketoximes using trifluoromethanesulphonic anhydride†
Abstract
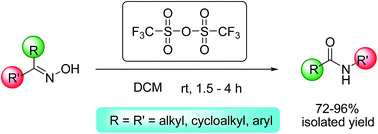
Trifluoromethanesulphonic anhydride (triflic anhydride: TA) has been successfully used as a reagent for Beckmann rearrangement in the conversion of a variety of ketoximes into amides without any additive or base. This reagent works well for the synthesis of a library of amides with excellent yields.

 Please wait while we load your content...
Please wait while we load your content...