Molecularly imprinted fluorescent chemosensor synthesized using quinoline-modified-β-cyclodextrin as monomer for spermidine recognition†
Abstract
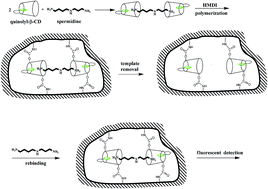
Polyamines are polycationic amines and playing important functions in the cellular growth and proliferation. Abnormal levels of polyamines in the biological fluids have been related to different diseases including cancers. However, polyamine analysis is a difficult task because there are no chromophores in the polyamine structures. In this study, a novel molecularly imprinted fluorescent chemosensor for spermidine detection has been synthesized using quinoline modified-β-cyclodextrin as the functional monomer. The imprinted receptors were formed by the interaction between the spermidine and β-cyclodextrin (β-CD). The fluorescence of the chemosensor has shown a “Turn-on” response mode which resulted from the increase of the environmental hydrophobicity around the quinoline group due to the inclusion of spermidine in the CD cavity. The chemosensor has selectivity for the spermidine and its structural analogue spermine due to the imprinting effect. In the research, the binding constant of the imprinted membrane was evaluated and the binding mechanism of the MIP was studied by 2D 1H NMR experiment. The research on spermidine analysis in serum demonstrated the imprinted chemosensor has good application potential in the biological sample analysis.

 Please wait while we load your content...
Please wait while we load your content...