A conjugated ketone as a catalyst in alcohol amination reactions under transition-metal and hetero-atom free conditions†
Abstract
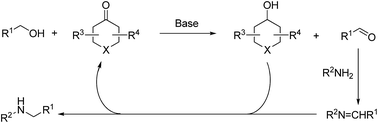
Here, we show the results of a molecular-defined conjugated ketone catalyzed alcohol amination reaction. Under the optimized reaction conditions, the yields to the desired products reached 98%. The reaction mechanism and kinetic study supposed that carbonyl–hydroxyl groups are the catalytically active sites, and the transfer-hydrogenation reactions progress via the recycling of carbonyl and hydroxyl groups. The catalytic process shows promise as an efficient and economic route for alcohol amination reactions.

 Please wait while we load your content...
Please wait while we load your content...