New insights into structure–activity relationships for propane hydrogenolysis over Ni–Cu bimetallic catalysts
Abstract
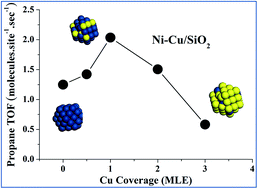
Propane hydrogenolysis has been investigated on Ni–Cu/SiO2 model catalysts under elevated pressure conditions. The surface Ni active sites on Ni–Cu/SiO2 were measured by selective hydrogen adsorption under ultrahigh vacuum (UHV) conditions. The specific activity of propane hydrogenolysis shows a slight increase and then decrease with increasing Cu coverages on Ni–Cu/SiO2 bimetallic catalysts, and varies within the 0.6 s−1 to 2.0 s−1 range. On the contrary, many previous studies show a three-to-five order decrease in the specific activity of ethane or propane hydrogenolysis over Ni–Cu bimetallic catalysts. The significant difference was explained by the possible over counting of active Ni sites in the selective hydrogen chemisorptions under atmospheric pressure conditions due to hydrogen spillover from Ni to Cu. New structure–activity relationships for Ni–Cu bimetallic catalysts in propane hydrogenolysis were established based on the present work. Furthermore, possible carbon deposits in this reaction were examined by post reaction Auger electron spectroscopy.

 Please wait while we load your content...
Please wait while we load your content...