Catalytic oxidation of 1,2-dichloroethane over Al2O3–CeO2 catalysts: combined effects of acid and redox properties†
Abstract
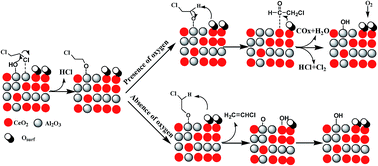
The lower temperature catalytic combustion of 1,2-dichloroethane (DCE) over Al2O3, CeO2 and Al2O3–CeO2 catalysts were studied. An apparent deactivation for pure Al2O3 and CeO2 catalyst was observed at 300 °C, which was ascribed to the formation of coke for pure Al2O3 while the strong adsorption of Cl species at active sites for pure CeO2. However, Al2O3–CeO2 mixed oxides exhibited a high stable activity compared with the pure Al2O3 and CeO2, which indicated that suitable acidity and excellent redox performance was equally important for total oxidation of DCE. TPSR experiments indicated that ClCH2CHO was the intermediate product and vinyl chloride (VC) was the main by-product for DCE catalytic oxidation over Al2O3–CeO2 catalyst, ClCH2CHO and VC were the competitive products of the C–H-scission ethoxides, which generated by α- and β-CH-scission respectively. Based on in situ DRIFTS study, a simple reaction pathway was proposed: Firstly, DCE adsorbed on surface hydroxyl groups or Lewis acid sites via the C–Cl bond and was attacked by the basic site (O2−), and then the intermediates such as aldehyde species and by-product VC formed. Sequentially, the aldehyde species were converted into H2O, COx and HCl via further Cl abstraction and oxidation process, by contrast, VC was not easy to be further decomposed because of its stable structure, the weak adsorption and difficult dissociation on acid sites.

 Please wait while we load your content...
Please wait while we load your content...