Click synthesis of ionic strength-responsive polyphosphazene hydrogel for reversible binding of enzymes†
Abstract
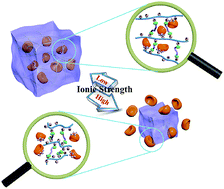
In this study, a chemically crosslinkable cationic polyphosphazene was synthesized and fabricated into ionic strength-responsive hydrogels for enzyme binding. This novel polyphosphazene was synthesized via the macromolecular substitution reaction of poly(dichlorophosphazene) with 2-dimethylaminoethylamine, followed by the quaternization to yield the allyl groups. Hydrogels were easily prepared via the thiol–ene click reaction between polyphosphazene and poly(ethylene glycol) dithiol (dithiol PEG) under UV radiation. The inner three-dimensional structure of the hydrogels was investigated by swelling experiments, mechanical property tests, and field emission-scanning electron microscopy. The resulting hydrogels exhibited sensitivity to the ionic strength of the surrounding environment. Lipase from Candida rugosa was selected as the model enzyme for the entrapment in these hydrogels, resulting in a maximum enzyme loading of 16.6 mg g−1 and activity retention as high as 61.6%. Furthermore, the cationic hydrogels were effectively used for reversible enzyme binding owing to the electrostatic interaction, regulated by the ionic strength.

 Please wait while we load your content...
Please wait while we load your content...