Pd-catalyzed decarboxylative allylic coupling of acetates of Baylis–Hillman alcohols with propiolic acids: a highly regio- and stereoselective synthesis of 1,5-diarylpent-1-en-4-yne derivatives†
Abstract
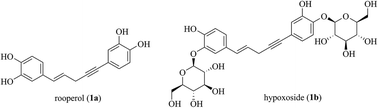
Pd-catalyzed decarboxylative allylic coupling of acetates of Baylis–Hillman alcohols with alkynyl carboxylic acids leading to the formation of an important class of 1,5-diarylpent-1-en-4-ynes in a highly regio- and stereoselective manner has been developed. Decarboxylative coupling happened via an exclusively SN2′ pathway. Acetates of the Baylis–Hillman alcohols derived from alkyl acrylates, ethyl vinyl ketone and phenyl vinyl sulfone provided exclusively (E)-1,5-diarylpent-1-en-4-ynes while the acetates of the Baylis–Hillman alcohols derived from acrylonitrile provided exclusively (Z)-1,5-diarylpent-1-en-4-ynes.

 Please wait while we load your content...
Please wait while we load your content...