Competitive biosorption of Cr(vi) and Zn(ii) ions in single- and binary-metal systems onto a biodiesel waste residue using batch and fixed-bed column studies†
Abstract
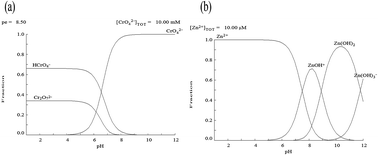
A feasible biosorption process for the removal of Cr(VI) and Zn(II) ions from single and binary solutions onto a defatted pongamia oil cake (DPOC) was investigated. The maximal biosorption capacities of Cr(VI) and Zn(II) ions in single metal solutions were found to be 166.60 mg g−1 and 123.45 mg g−1, respectively. Due to the internal competition effect, the biosorption capacity of Cr(VI) and Zn(II) ions in the binary system was reduced to 125.10 mg g−1 and 83.30 mg g−1, respectively. Experimental data were well described by Freundlich isotherm. Kinetic studies were also preformed and the rate kinetics was followed with pseudo-second order model. Thermodynamic parameters such as Gibbs free energy (ΔG°), entropy (ΔS°) and enthalpy (ΔH°), were estimated in both single and binary systems, which showed that biosorption on the DPOC was of exothermic nature. In the column study, biosorption of metals using single and binary solutions was fitted well by the Thomas model.

 Please wait while we load your content...
Please wait while we load your content...