Preparation and characterization of layered LiNi0.9Co0.05Mn0.025Mg0.025O2 cathode material by a sol–gel method for lithium-ion batteries
Abstract
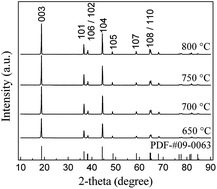
LiNi0.9Co0.05Mn0.025Mg0.025O2 was prepared by a sol–gel method using citric acid as a chelating agent. Calcination temperature and calcination time played a critical role in the preparation of the materials, and their effects on the properties of the materials were discussed in detail. The optimal calcination temperature and time were determined to be 700 °C and 12 h, respectively. The sample prepared under the above optimal conditions had a well ordered hexagonal layered structure. The charge–discharge tests showed that the initial capacities of the sample were 201.0 mA h g−1 and 187.6 mA h g−1 at the discharge rate of 0.1 C and 1 C between 2.8 and 4.3 V, respectively. The capacity retention ratio was 99.3% at 0.1 C after 10 cycles and 91.86% at 1 C after 50 cycles. The excellent rate capability of the sample prepared at the optimal conditions was also observed.

 Please wait while we load your content...
Please wait while we load your content...