The effect of NaF on the electrochemical behavior of the Mg–11Li–3.5Al–1Zn–1Sn–1Ce–0.1Mn electrode in NaCl solution
Abstract
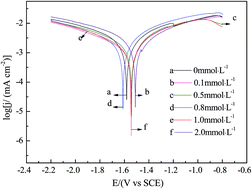
In order to improve the electrochemical behavior of the Mg–11Li–3.5Al–1Zn–1Sn–1Ce–0.1Mn electrode in a 0.7 mol L−1 NaCl solution, different concentrations of sodium fluoride (NaF) as the electrolyte additive are added into the electrolyte solution and their effects on the electrochemical performances of the electrode are investigated by the methods of potentiodynamic polarization, potentiostatic oxidation, electrochemical impedance spectroscopy (EIS) and scanning electron microscopy (SEM) with EDS analysis. It is found that the corrosion current density of the electrode decreases with the concentration of NaF in the following order: 0.5 mmol L−1 > 0.1 mmol L−1 > 1.0 mmol L−1 > 2.0 mmol L−1 > 0.8 mmol L−1 > 0 mmol L−1. The discharge current density of the electrode in the electrolyte solution containing 0.8 mmol L−1 NaF is higher than that in the other concentrations at the discharging potentials of −0.8 V, −1.0 V and −1.2 V. The electrode in the electrolyte solution containing 0.8 mmol L−1 NaF retains a larger reaction surface area during discharge, which leads to the highest discharge activity. The different concentrations of NaF in the 0.7 mol L−1 NaCl electrolyte solution can change the electrochemical behavior of the Mg–11Li–3.5Al–1Zn–1Sn–1Ce–0.1Mn electrode, and the optimum concentration of the electrolyte additive NaF is 0.8 mmol L−1. The Mg–H2O2 semi-fuel cell with the Mg–11Li–3.5Al–1Zn–1Sn–1Ce–0.1Mn anode presents a maximum peak power density of 77 mW cm−2 when it is measured in a 0.7 mol L−1 NaCl solution containing 0.8 mmol L−1 NaF as the anolyte at room temperature, which is higher than that measured in a 0.7 mol L−1 NaCl solution as the anolyte (62 mW cm−2). NaF is an effective anolyte additive for the Mg–11Li–3.5Al–1Zn–1Sn–1Ce–0.1Mn alloy electrode in Mg–H2O2 semi-fuel cells.

 Please wait while we load your content...
Please wait while we load your content...