On the mechanism of the Shapiro reaction: understanding the regioselectivity†
Abstract
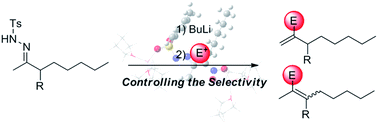
A detailed DFT-level mechanism elucidation of the two-step reaction of tosylhydrazones with alkyllithium reagents (the Shapiro reaction) is presented. A rationale of the experimental regioselectivity is offered together with some suggestions for modifying the experimental main regioisomer. Also, the proposed general mechanism was checked with a recent modification of the Shapiro reaction involving a fluorination reaction.

 Please wait while we load your content...
Please wait while we load your content...