New insight into the promotion effect of Cu doped V2O5/WO3–TiO2 for low temperature NH3-SCR performance†
Abstract
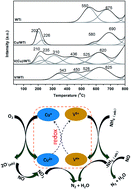
The promotion effect of Cu on the V/WTi catalyst for the selective catalytic reduction of NOx by NH3 was investigated in the temperature range of 150–400 °C. The Cu addition shows a superior NH3-SCR performance in comparison with the V/WTi sample. The catalysts were characterized by XRD, Raman, EPR, H2-TPR, XPS, and in situ DRIFTS techniques. The obtained results reveal that the Cu oxides in close proximity to V oxides on the surface facilitate the formation of double redox couples of V5+/V4+ and Cu2+/Cu+, which may play a critical role in the superior NH3-SCR performance. The electronic interactions caused by the redox cycle of Cu2+ + V4+ ↔ V5+ + Cu+ could significantly improve the redox properties of the vanadium species, which is beneficial for the activation of NH3 species bound to the vanadium species. Moreover, the redox cycle of Cu2+ + V4+ ↔ V5+ + Cu+ induces the formation of high-activity nitrate species adsorbed on Cu species. The kinetic analysis reveals that the Cu doping induces the decrease of the activation energy (Ea) of NH3-SCR.

 Please wait while we load your content...
Please wait while we load your content...