Triple bond-modified anthracene sensitizers for dye-sensitized solar cells: a computational study†
Abstract
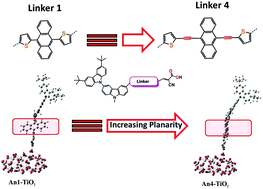
We performed a theoretical investigation on a series of organic dyes incorporating an anthracene moiety between a carbazole donor group and a cyanoacrylic acid acceptor, in which a triple bond (TB)-modified moiety acts as a π-conjugated linker. Density functional theory (DFT) and time-dependent DFT (TD-DFT) were applied to understand the electronic, photophysical, and electron injection properties of the dyes. We found that optimized anthracene structures lay almost perpendicular to the plane of the adjacent substituents. The introduction of a modified TB moiety significantly decreases the dihedral angle and results in a planar structure, which extends the length of the π-conjugated system to provide a broader absorption spectrum, the An4 dye exhibited the greatest red shift of the maximum absorption wavelength. Introduction of a TB moiety into the dye structure facilitates electron transfer from the donor and acceptor. The TB-modified dye structure has a significant effect on electron injection from the dye sensitizer to the TiO2 surface. Our results demonstrate that use of computational design can to help the experimentalist for looking out for future developments to identify TB-modified anthracene sensitizers for highly efficient solar cells.

 Please wait while we load your content...
Please wait while we load your content...