PEG-assisted hydrothermal synthesis and electrochemical performance of ZnO/Ketjenblack nanocomposite for lithium ion batteries
Abstract
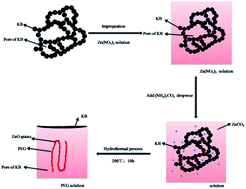
ZnO/Ketjenblack(KB) composite was fabricated by means of a facile PEG-assisted hydrothermal synthesis process, characterized by X-ray powder diffraction, scanning electron microscopy, field emission transmission electron microscopy, thermogravimetric analysis, nitrogen sorption, energy dispersion spectroscopy, galvanostastic charge/discharge test, cyclic voltammogram and electrochemical impedance spectroscopies. The results show that the composite forms a special porous structure with ZnO particles embedded in the mesopores of Ketjenblack, which favors the improvement of electrochemical performance. Compared with unmodified ZnO, ZnO/KB composite exhibits superior electrochemical performances. ZnO/KB composite delivers a discharge capacity of 418.9 mA h g−1 at the discharge density current of 800 mA g−1, whereas the ZnO only gives 116.1 mA h g−1. Moreover, the sample retains a discharge capacity of 538.4 mA h g−1 after 100 cycles at a current density of 100 mA g−1. The improved electrochemical performance can be ascribed to the combined Ketjenblack, which serves as conducting buffering matrix during lithiation/delithiation process.

 Please wait while we load your content...
Please wait while we load your content...