3-Substituted 6-oxoverdazyl bent-core nematic radicals: synthesis and characterization†
Abstract
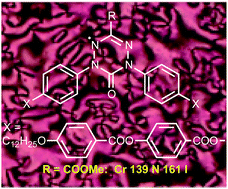
A series of bent-core derivatives of 6-oxoverdazyl 1[12] was synthesized and mesogenic properties were investigated in the pure form and in binary mixtures. Results demonstrate that the effectiveness of the C(3) substituent in nematic phase stabilization follows the order: COOMe > m-FC6H4 > Ph > thienyl > o-FC6H4, which is consistent with steric parameters established with DFT computational methods and opposite to the order of the appearance of a re-entrant isotropic phase. The effect of alkyl chain elongation on mesogenic properties and the effect of C(3) substituent on electronic absorption spectra are also investigated.

 Please wait while we load your content...
Please wait while we load your content...