Investigation of the synthesis and properties of isophorone and ether units based semi-aromatic polyamides
Abstract
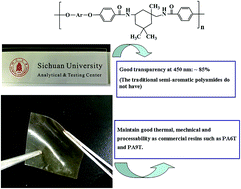
The difluoro-substituted monomer, N,N′-bis(4-fluorobenzoyl) isophorone diamine (BFID), was prepared via an interfacial reaction from isophorone diamine and 4-fluorobenzoic chloride. It was then reacted with hydroquinone (or resorcinol, 1,1-bis(4-hydroxyphenyl)-1-phenylethane (BHPPE)) to yield a series of semi-aromatic polyamides. For the synthesized semi-aromatic polyamides, differential scanning calorimetry and thermogravimetric analysis confirmed their high glass transition temperatures (Tg), which were between 217 and 239 °C, and good thermal stability with initial degradation temperatures (Td) in the range of 425–430 °C. Tensile test and dynamic mechanical analysis (DMA) results revealed the good mechanical properties of the semi-aromatic polyamides at ambient temperature and even at 200 °C. Rheological characterization displayed complex viscosities at 310 °C for the semi-aromatic polyamide in the range of 990–1350 Pa s, which suggests that they are suitable for melt processing. Better solubility properties for the resultant semi-aromatic polyamides compared to commercial ones were also found. All the results indicated the good processability of the synthesized semi-aromatic polyamides. In addition, they were found to have almost identical optical transmittance (Tran%: 83–85% at 450 nm) as polycarbonate (PC), in the UV-visible region.

 Please wait while we load your content...
Please wait while we load your content...