Room-temperature phosphorescence by Mn-doped ZnS quantum dots hybrid with Fenton system for the selective detection of Fe2+†
Abstract
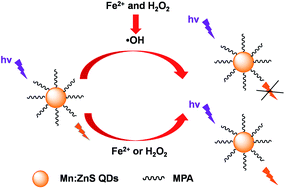
The phosphorescent 3-mercaptopropionic acid (MPA) capped Mn-doped ZnS quantum dots (MPA–Mn:ZnS QDs)–Fenton hybrid system was developed for a highly sensitive detection of Fe2+ in environmental samples and biological fluids. The phosphorescence of the MPA–Mn:ZnS QDs can be effectively quenched by hydroxyl radicals produced from the Fenton reaction between Fe2+ and H2O2 at a low concentration level. However, the phosphorescence of the MPA–Mn:ZnS QDs cannot be quenched by either Fe2+ or H2O2 individually at the same concentration level. Thus, Fe2+ can be indirectly detected using the phosphorescence quenching caused by hydroxyl radicals based on the Fenton reaction. A possible mechanism for the quenching effect of Fe2+ was elucidated as electron transfer from the conduction band of the MPA–Mn:ZnS QDs to the unoccupied band of hydroxyl radicals. The phosphorescent hybrid system allowed a highly sensitive detection of Fe2+ in an aqueous solution with a wide linear range of 0.01–10 μM and a detection limit of 3 nM, and the precision for 11 replicate detections of 0.1 μM Fe2+ was 1.5% (relative standard deviation, RSD). The developed method was applied to determine Fe2+ in environmental samples and biological fluids with quantitative spike recoveries from 95% to 104%.

 Please wait while we load your content...
Please wait while we load your content...