Hydrogen bond breaking of TPU upon heating: understanding from the viewpoints of molecular movements and enthalpy
Abstract
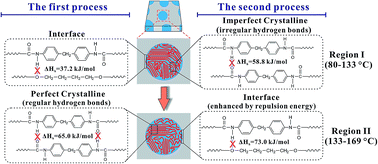
Hydrogen bond breaking of TPU based on 4,4′-methylenediphenyl diisocyanate (MDI)/1,4-butanediol (BDO) upon heating was studied and elucidated from molecular movements and enthalpy. Two temperature regions of hydrogen bond breaking, region I (80–133 °C) and region II (133–169 °C), were determined via the combination of PCMW2D correlation with FTIR and DSC. The method of calculating the enthalpy of the hydrogen bond breaking was established via Van't Hoff plots. We also proposed a method of calculating the relative content of different hydrogen bonds. In region I, ΔHh = 58.8 ± 0.5 kJ mol−1 for N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and ΔHh = 37.2 ± 0.4 kJ mol−1 for N–H and C–O–C groups. The content of hydrogen bonds generated by N–H and C
O, and ΔHh = 37.2 ± 0.4 kJ mol−1 for N–H and C–O–C groups. The content of hydrogen bonds generated by N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O is 88.4%, and that of N–H and C–O–C is 11.6%. In region II, ΔHh = 65.0 ± 1.1 kJ mol−1 for N–H and C
O is 88.4%, and that of N–H and C–O–C is 11.6%. In region II, ΔHh = 65.0 ± 1.1 kJ mol−1 for N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and ΔHh = 73.0 ± 3.9 kJ mol−1 for N–H and C–O–C groups. The contents of these two hydrogen bonds are 71.2% and 28.8%, respectively. The surprisingly high value of ΔHh = 73.0 ± 3.9 kJ mol−1 for N–H and C–O–C in region II is actually due to the stabilizing effect of the repulsion energy on hydrogen bonds at the interface. 2D correlation analysis was used to investigate the sequential order of the groups' movement involved in hydrogen bond breaking. In region I, the breaking of a small amount of hydrogen bonds between N–H and C–O–C at the interface first occurs, and then the breaking of irregular hydrogen bonds between N–H and C
O, and ΔHh = 73.0 ± 3.9 kJ mol−1 for N–H and C–O–C groups. The contents of these two hydrogen bonds are 71.2% and 28.8%, respectively. The surprisingly high value of ΔHh = 73.0 ± 3.9 kJ mol−1 for N–H and C–O–C in region II is actually due to the stabilizing effect of the repulsion energy on hydrogen bonds at the interface. 2D correlation analysis was used to investigate the sequential order of the groups' movement involved in hydrogen bond breaking. In region I, the breaking of a small amount of hydrogen bonds between N–H and C–O–C at the interface first occurs, and then the breaking of irregular hydrogen bonds between N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the TPU hard blocks dominates, resulting in the melting of the imperfect crystallinity in the hard blocks. In region II, the breaking of regular hydrogen bonds between N–H and C
O in the TPU hard blocks dominates, resulting in the melting of the imperfect crystallinity in the hard blocks. In region II, the breaking of regular hydrogen bonds between N–H and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the perfect crystalline of the hard blocks first occurs, and is then followed by hydrogen bond breaking of N–H and C–O–C enhanced by the repulsion energy at the interface, leading to the order–disorder transition (ODT) of TPU.
O in the perfect crystalline of the hard blocks first occurs, and is then followed by hydrogen bond breaking of N–H and C–O–C enhanced by the repulsion energy at the interface, leading to the order–disorder transition (ODT) of TPU.

 Please wait while we load your content...
Please wait while we load your content...