Quantitative analysis of the relationship between the dispersion stability of mixed-surfactant Ag nanoparticles and their composition†
Abstract
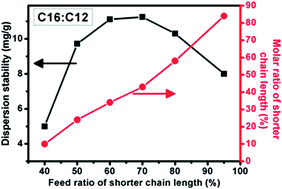
Understanding the surfactant layer composition and morphology of metal nanoparticles (NPs) with single-molecule surfactants is crucial for controlling the properties of NPs. Previously, we reported that the dispersion stability of Ag NPs could be greatly enhanced by mixing n-alkyl carboxylic acid surfactants with two different chain lengths. In this paper, we continued this previous study by quantitatively analyzing the relationship between the surfactant shell composition and the dispersion stability of mixed-surfactant Ag NPs. We found that the Ag NP dispersion stability was greatly influenced by the molar ratio and the difference in chain lengths of the surfactants. To obtain a superior dispersion stability, the molar ratio of the two surfactants with different chain lengths should be close to 1 : 1, and the difference in chain lengths should be greater than or equal to four carbon atoms. We also found that the dispersion stability of Ag NPs with a mixed surfactant of palmitic acid (C16) and dodecanoic acid (C12) is insensitive to the type of solvent used.

 Please wait while we load your content...
Please wait while we load your content...