Donor–acceptor copolymers containing the phthalazinone–thiophene structure synthesized by classical nucleophilic aromatic polymerization†
Abstract
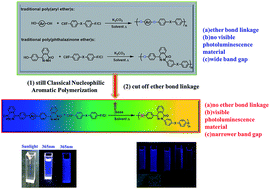
A series of new donor–acceptor copolymers based on a novel monomer containing phthalazinone and thiophene structures, were synthesized by classical nucleophilic displacement step polymerization reaction, and then were fully characterized. Their thermal, optical, electrochemical properties and quantum chemical calculations were investigated in detail. The comparisons between the linkage of ketone/di-ketone/sulfone groups and phenyl/naphthalene groups in the resulting polymer backbone were carried out to explore the relationship of the structure and the properties in the polymer system. The number average molecular weights of the resulting copolymers are 15 kDa–24 kDa. All of the copolymers are stable up to 441 °C, and their 5% weight loss temperatures are higher than 489 °C. Their optical band gap and HOMO energy levels vary from 2.36 eV to 2.76 eV and −5.16 eV to −5.51 eV, respectively. The soluble copolymers obtained exhibit blue-fluorescence. Their photoluminescence quantum yield ranges from 0.04 to 0.28 in N-methyl-2-pyrrolidone. These non-ether bond polymers may be potential candidates in photoelectric application.

 Please wait while we load your content...
Please wait while we load your content...